Injeq is happy to announce that Injeq IQ-Tip device with its indications for use meet the criteria and have been granted designation as a Breakthrough Device by the U.S. Food and Drug Administration (FDA).
Injeq CEO Timo Hänninen said: “We are excited about this recognition from the FDA. The already over a decade continued engineering development work with scientific clinical research is now paying off. First Injeq products were approved by European authorities in 2021 with the CE mark under the strict MDR requirements, and now the US medical device authorities are expressing their willingness to assist our market entry with expert assistance and accelerated processing for the US marketing authorization.”
Injeq product
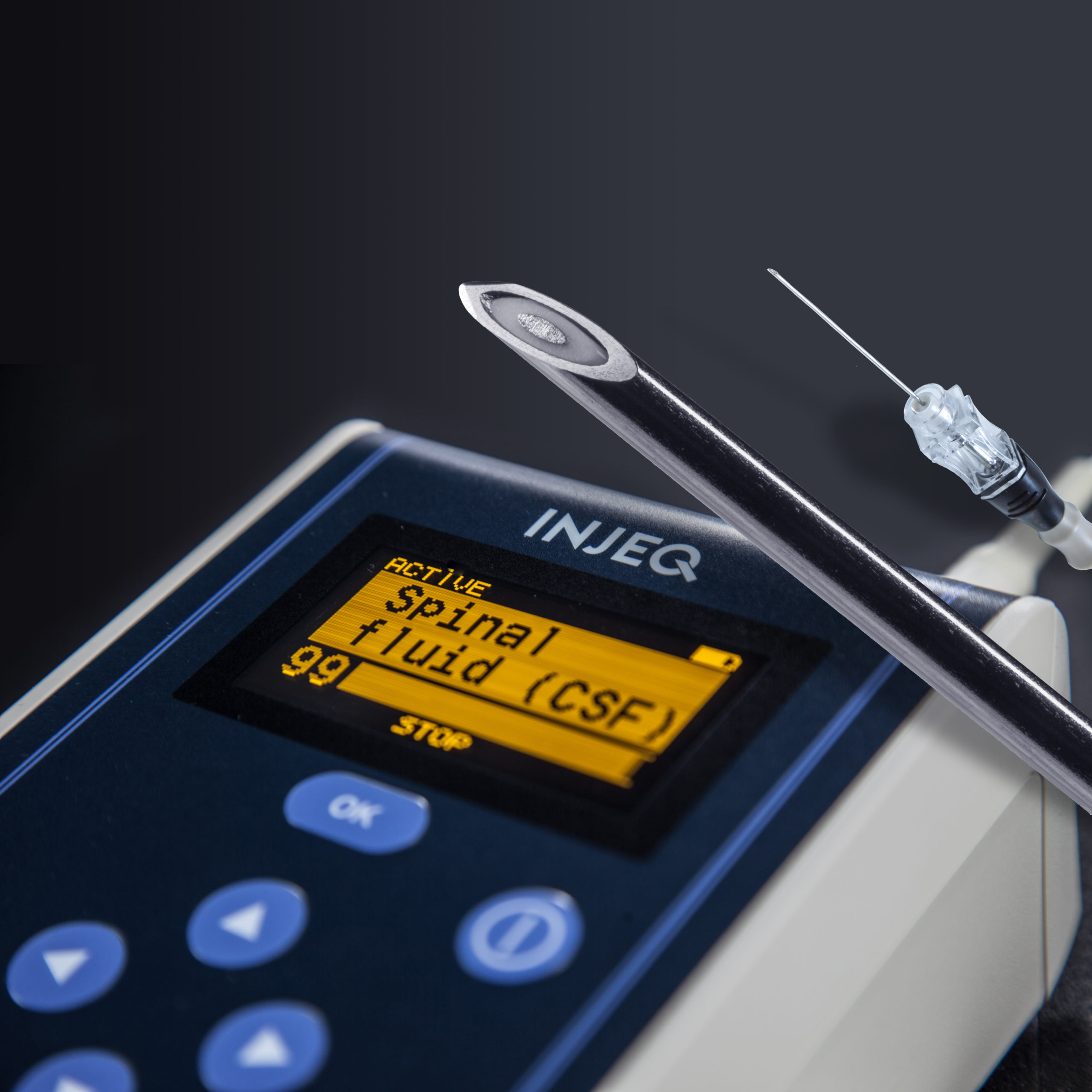
Injeq IQ-Tip® system facilitates lumbar puncture by alerting the physician with an audio alarm when the spinal fluid has been detected at the tip of the needle. This bioimpedance spectroscopy based cerebrospinal fluid detection allows the specialist performing the lumbar puncture to also interpret from the conductivity index on the analyzer display in real time where the needle tip is advancing in the lumbar area.
The main advantages, however, seem to be on the patients’ side. The observations from several clinical studies show a remarkably smaller incidence of the most common complications related to lumbar punctures such as post dural puncture headache and traumatic (blood containing) lumbar punctures compared to the lumbar punctures done with the same size conventional needles.
What FDA Breakthrough Device designation means
The program is a voluntary program for certain medical devices that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. Injeq products offer possibly lifesaving diagnostics and treatment punctures with leukemia patients. In several neurological diseases Injeq products can provide more reliable diagnostics with good quality cerebrospinal fluid samples which can save time and money in healthcare. Also, the less complications there are from lumbar punctures the less there is human suffering and the more there can be monetary savings.
The goal of the Breakthrough Devices Program is to provide US patients and healthcare providers with timely access to these new medical devices by speeding up their assessment, and review process with US authorities. The Breakthrough Devices Program offers manufacturers an opportunity to interact with the FDA’s experts and receive feedback from the FDA. The manufacturer can request for a Sprint Discussions with FDA experts, so that certain topics like regulatory interpretations get quickly clear and the manufacturer’s processes can continue smoothly. Also, FDA offers a Clinical Protocol Agreement which is binding for both sides. This guarantees that the required clinical studies in US will be sufficient by one go. Manufacturers can also expect prioritized review of their submission.
Investment round open
Injeq currently has an open investment round in Invesdor crowdfunding platform. Direct link to Injeq round at Invesdor:
About Injeq
Injeq Plc is a Finnish medical technology company founded in 2010 by a team of clinicians and engineers who innovated a real-time cerebrospinal fluid detection method to assist physicians to perform blind lumbar punctures.
Injeq IQ-Tip® system is CE marked under European Medical Device Requirements, and the company has distributors in 14 countries across Europe and additionally in Kuwait, Taiwan and Hong Kong area. Injeq has plans to expand its distributor network globally in the EMEA, Americas and APAC regions.