There are two weeks left in Injeq’s share issue. Read the promising news and invest:
Funding: Injeq Oyj (invesdor.com)
The way to go to the German market
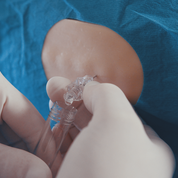
A major neurological research hospital in Erlangen has expressed their interest to study lumbar punctures with Injeq IQ Tip system. Discussion has been about comparing Injeq smart needle to the atraumatic needle that is commonly used in Erlangen. So far, in earlier Injeq studies the observations present almost half smaller post puncture headache incidence than lumbar punctures done with atraumatic needles. See the picture.
Progress in FDA Breakthrough Device designation
During July Injeq has discussed with US authorities about Breakthrough Device designation in good spirit. We are expecting the results of the evaluation next week. The designation would ensure smooth processing for the Injeq products’ US market approval. Fingers cross!